The Role of Epinephrine Auto Injectors in Expanding the Anaphylaxis Treatment Market
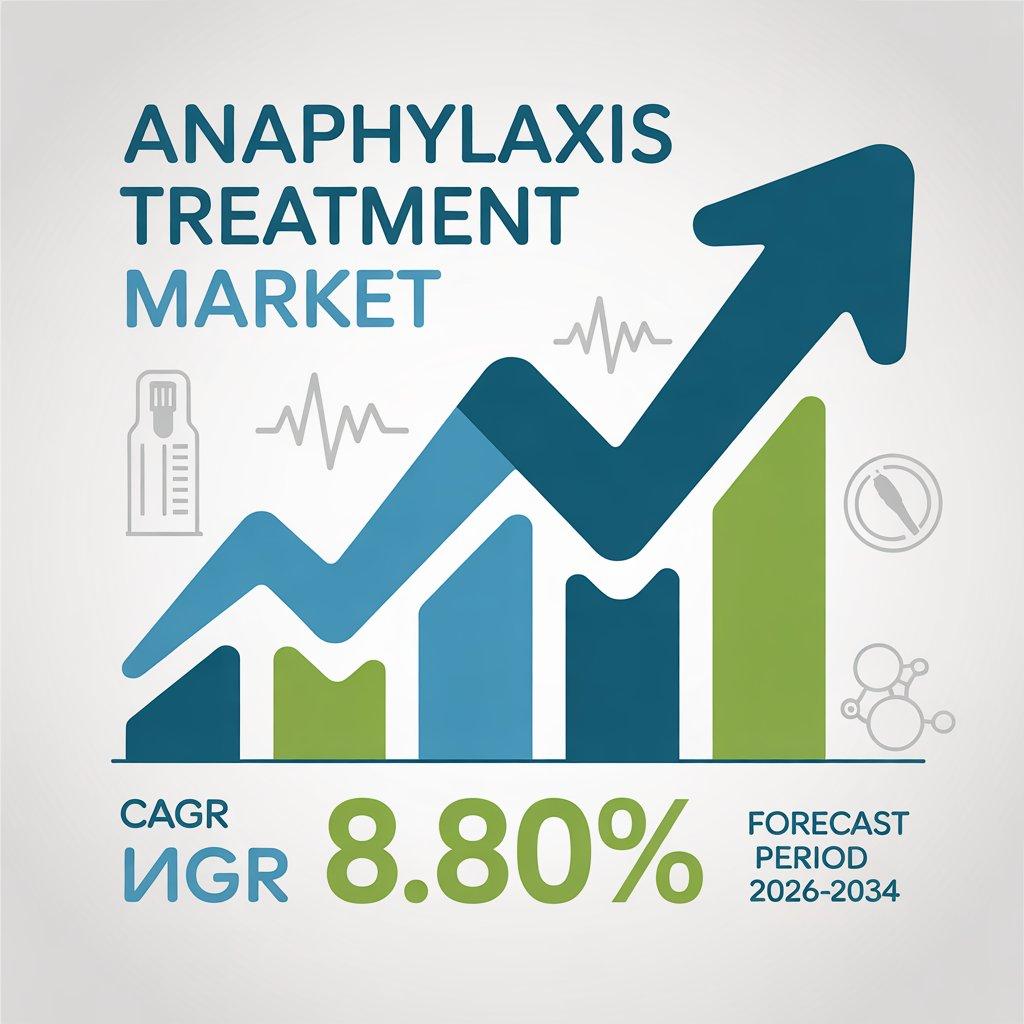
Epinephrine auto injectors play a central role in the expansion of the global anaphylaxis treatment market, acting as both the clinical standard of care and the primary commercial growth driver. As highlighted in the given content, anaphylaxis is a rapid onset and potentially fatal condition that requires immediate intervention. The ability of auto injectors to deliver epinephrine quickly, safely, and outside of hospital settings has fundamentally reshaped emergency allergy management and significantly increased market demand.
Epinephrine as the foundation of anaphylaxis treatment
Epinephrine is universally recognized as the first line treatment for anaphylaxis due to its rapid vasoconstrictive, bronchodilatory, and cardiovascular stabilizing effects. Clinical guidelines across global healthcare systems emphasize early epinephrine administration as the single most important factor in preventing fatal outcomes. This strong clinical consensus anchors epinephrine products at the core of the treatment market, ensuring consistent and recurring demand.
Auto injectors amplify the value of epinephrine by addressing real world challenges associated with emergency use. During an anaphylactic episode, patients often experience panic, impaired breathing, and loss of coordination. Auto injectors simplify administration by combining pre measured dosing with intuitive activation mechanisms, enabling use by patients, caregivers, teachers, and non medical bystanders. This usability advantage is a key reason why auto injectors dominate prescription volumes compared with traditional ampoules or manual syringes.
How auto injector design innovation drives market growth
Technological advancement in auto injector design has been a major force behind the market’s strong compound annual growth rate. As noted in the given content, manufacturers are continuously improving device features to reduce hesitation and increase confidence during emergency use. Innovations such as smaller and more discreet devices encourage patients to carry them at all times, which directly supports higher adoption rates.
Voice guided instructions and audible prompts address a critical behavioral barrier by guiding users step by step during stressful situations. Needle concealment reduces fear, particularly among children and caregivers, while improved temperature stability protects epinephrine efficacy across varied climates and storage environments. These enhancements do not merely improve convenience. They expand the practical use cases of auto injectors, making them suitable for schools, workplaces, travel, and community emergency kits.
Rising awareness and preparedness amplify demand
The growing emphasis on allergy awareness and emergency preparedness strongly reinforces auto injector demand. Public health campaigns, patient advocacy groups, and pharmaceutical companies have increased education around recognizing anaphylaxis symptoms and responding immediately. As awareness improves, more individuals are diagnosed as high risk and more physicians proactively prescribe epinephrine auto injectors.
Preparedness standards are also evolving at the institutional level. Schools, childcare facilities, and public venues increasingly maintain stocked auto injectors as part of emergency protocols. This shift transforms auto injectors from purely individual prescriptions into essential community health tools, expanding distribution volumes through hospital pharmacies, retail channels, and government procurement programs.
Get a Sample PDF: https://www.theinsightpartners.com/sample/TIPRE00022791
Impact on regional and emerging markets
Auto injectors are particularly influential in expanding the anaphylaxis treatment market in emerging regions. As healthcare infrastructure improves in Asia Pacific and Latin America, diagnosis rates for food and drug allergies are rising. Auto injectors provide a scalable solution that does not depend on immediate hospital access, making them highly attractive in regions with variable emergency response times.
The introduction of generic auto injectors and affordability focused strategies further accelerates adoption in these markets. By reducing cost barriers, manufacturers enable wider access while maintaining clinical effectiveness, which supports sustained volume growth.
Key players driving the auto injector segment
- Amneal Pharmaceuticals Inc.
- Mylan N.V. (Viatris)
- Teva Pharmaceutical Industries Ltd.
- Adamis Pharmaceutical Corporation
- Pfizer Inc.
- Abbott
Future Outlook
Epinephrine auto injectors will remain the primary growth engine of the anaphylaxis treatment market throughout the forecast period. Continued innovation aimed at usability, affordability, and stability will further broaden adoption across both developed and emerging regions. At the same time, next generation delivery concepts such as non needle formulations may complement rather than replace auto injectors, expanding the overall treatment ecosystem. As allergy prevalence continues to rise globally, auto injectors will play an increasingly critical role in shaping preparedness standards, market expansion, and long term growth.
- الاقتصاد والتجارة
- فن
- كورسات
- الحرف اليدوية
- الطعام والشراب
- الألعاب والترفيه
- الصحة
- تكنولوجيا
- أخرى
- دين
- رياضة